Genetic engineering of T lymphocytes can transform them into powerful innovative anticancer drugs. For over 25 years, based on advances in cell engineering and process manufacturing, T-cell therapy has dramatically improved the efficacy of patients with refractory cancer. One of the most striking examples is the CD19 CAR-T therapy. CAR-T therapy is the expression of chimeric antigen receptor (CAR) on the surface of T cells, which changes the specificity and function of lymphocytes. CAR targeting CD19 has shown good efficacy in treating blood cancer caused by B cell carcinogenesis. Kite Pharma and Novartis' CD19 CAR-T cell therapy are expected to be approved by the FDA this year. The success of CD19 CAR-T therapy in some leukemias and lymphomas is expected to apply CAR-T cell therapy to other malignant tumors. A range of novel target antigens and CAR designs are under development and will be tested in blood and solid tumors. In addition, autologous T cells are currently the focus of cell-based cancer immunotherapy, but allogeneic cell sources, including stem cell-derived "off the shelf" T cells, will play an important role in the future. Recently, Nature magazine conducted an inventory of the current status and development direction of engineering T cell therapy. We bring a detailed interpretation in this article.
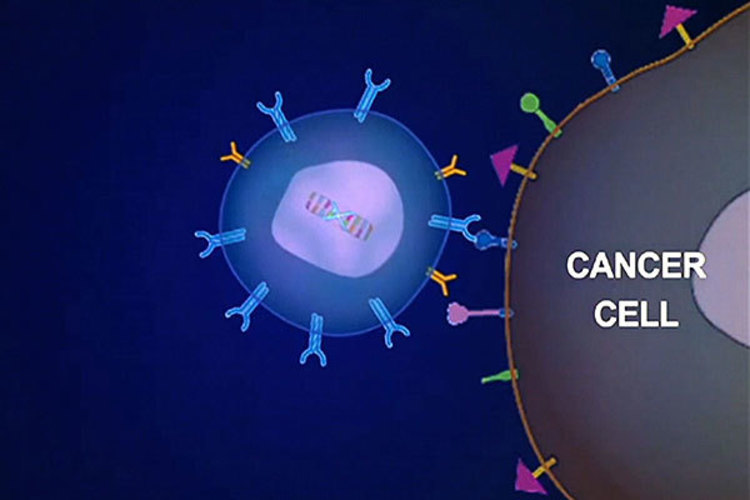
Engineering T cell principle
When T cells develop in the thymus, they acquire an antigen receptor called T cell receptor (TCR). The recognition of antigen by T cells under physiological conditions is accomplished by the TCR-CD3 complex. TCR consists of alpha and beta chains whose structure determines the antigen specificity of the TCR. CD3 includes four subunits, γ, δ, ε, and ζ, which function to initiate the T cell activation program.
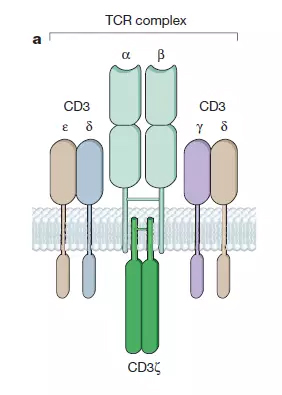
â–² T cell receptor (TCR) structure (Source: Nature)
The most natural way to engineer T cells is to use genetic engineering to express TCRs specific for the antigen in T cells, which, when complexed with CD3, are capable of conferring specific antigen specificity to T cells. The use of genetic engineering to express TCR in T cells can produce a large number of T cells that are identical in phenotype to the naturally occurring T cells of the body. These anti-cancer or anti-viral T cells can act to enhance the immune response in patients who are unable to produce sufficient immune responses to fight tumors or other diseases. The current implementation of this strategy focuses on isolating the TCR with the best specificity and affinity, designing molecular mechanisms to prevent cross-reactivity of TCR, and reducing mismatches between the alpha and beta chains. TCR-T cell therapy has shown significant anticancer effects in small clinical trials in patients with melanoma and sarcoma.
Another way for T cells to recognize new antigens is by designing artificial receptors for antigens. Such intracellular fragments of artificial receptors typically require a CD3 ζ chain to ensure that the receptor is capable of initiating a T cell activation program. The first generation of CAR design fused the CD3 ζ chain with antibody fragments that recognize the antigen, but this CAR design was not sufficient to stimulate a sufficiently strong immune response in the experiment. Current second generation CAR designs incorporate T cell co-stimulating signal receptors in artificial receptors. This combination of a T cell activating fragment (CD3z chain) and a costimulatory receptor fragment (most commonly CD28) on the same CAR allows T cells to recognize not only specific antigens but also interleukin-2 (interleukin- 2) and proliferate after repeated exposure to antigen. The second generation CAR design is currently the mainstream of CAR-T therapy.
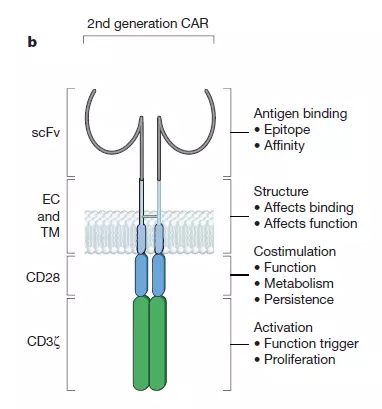
â–²Chimeric antigen receptor (CAR) structure (Source: Nature)
Second-generation CAR designs mostly use the single chain variable fragments (scFv) to identify antigens. There are currently more than 100 specific CARs, and at least eight co-stimulatory signal receptor fragments are used by researchers. The second-generation CAR design that was the most well-studied was the CARs using the CD28 and 4-1BB co-stimulation domains. Both types of designs have achieved significant clinical results in clinical trials for the treatment of recurrent B cell carcinogenesis. CD-28-based CARs promote active T cell proliferation, but T cell persistence is limited. The ability of 4-1BB-based CARs to induce effector T cells is relatively low, but supports longer T cell persistence.
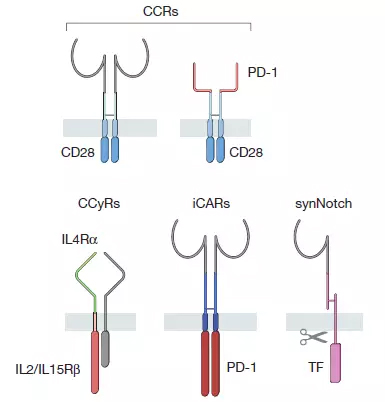
â–² CAR family synthetic receptor members (Source: Nature)
Skin Care Powder,Honeysuckle Extract,Honey Powder,Eriobotrya Japonica Leaf Extract
Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.sntbiology.com