What are the consequences of the question of the authenticity and integrity of the data? I am afraid there is no more suitable example than the CRO of the US CRO. In July 2011, the US Food and Drug Administration (FDA) clearly stated in a warning letter that the FDA has sufficient evidence that Cetero “modifies the test sample to meet pre-set criteriaâ€, “confirmed issues include Forgery of staff time and data records, and "pharmacists forged laboratory records in order to obtain higher pay."
After discovering these problems, the FDA notified the relevant pharmaceutical companies and biotech companies that if they wanted their products to be approved, they might have to re-do the relevant clinical studies or re-examine all the Houston clinical trials entrusted to Cetero. Whether the results of clinical trials conducted in the course meet the requirements for data integrity. Later, Cetero claimed that the involved personnel only included six employees in the company, and also successfully completed the rectification of the project in a shorter period of time than the specified deadline. However, Cetero's business was still hit hard. In the end, Cetero, which lost its trust in the industry, filed a bankruptcy application in March 2012.
The reason behind all of the above is obvious. The fundamental purpose of clinical trials is to gather data for the relevant decisions required for medical products. Therefore, the authenticity and integrity of the data is the core value of clinical trials. Obviously, if the data can support the security and effectiveness of this product, then it is more likely to be approved for listing. However, once the data is artificially affected or becomes untrustworthy, the safety and effectiveness of the product cannot be judged, and the probability of obtaining approval is greatly reduced. If clinical trials have such problems from the beginning, they will increase the time cost of redo testing, submitting rectification reports, and analysis, which may be months, or even years, and all of this could have been avoided.
However, it is difficult to know how to do it. People who are familiar with drug research and development know that to obtain real and complete data is inseparable from good experimental design, perfect quality control system, and technical, input and well-trained personnel support. And the sponsor must also take responsibility for internal quality control for the truth and integrity of the data, and choosing a reliable partner is one of its important responsibilities.
No record is equal to no occurrence
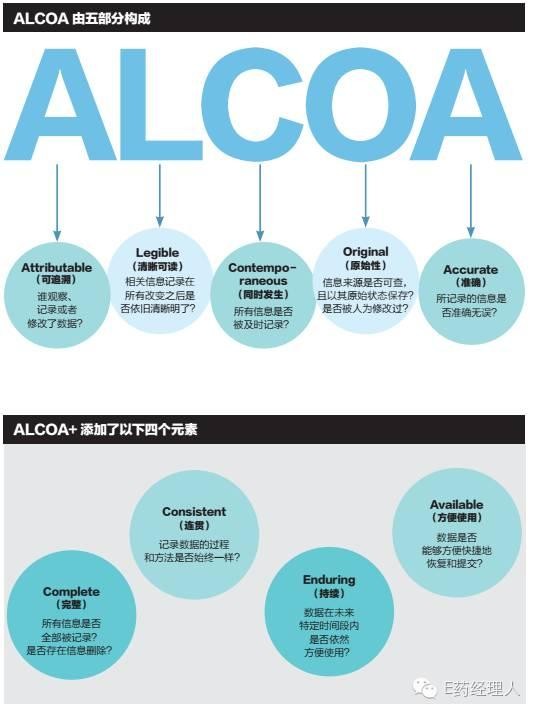
Every behavior in a clinical trial, including behavior that is not in a clinical setting, must be documented. There are two sentences that are particularly relevant: "No record is equal to no occurrence"; "Do not have to do it." These two sentences reflect the essence of the provisions of the International Drug Coordination (ICH) GCP on the principles of good recording. The whole process of clinical trials is not recorded in accordance with this spirit, and the authenticity and integrity of clinical trial data can only be discussed on paper. It should be pointed out that in addition to accurately and comprehensively recording the behavior of all clinical trials, there are four major requirements for the record of the data itself: accuracy, comprehensiveness, completeness, and traceability. And these requirements also apply to other data related to clinical trial data. In the United States, there is an abbreviation for the authenticity and integrity of data, ALCOA (or ALCOA+). This acronym was first proposed by Stan W. Wollen in his FDA law enforcement office in the 1990s.
Lab Water Distiller,Laboratory Distiller,Laboratory Water Distiller,Laboratory Water Distillation Equipment
ZHEJIANG FOMOS MEDICAL TECHNOLOGY CO.,LTD. , https://www.ifomos.com