A type of hemophilia gene therapy has a significant effect, significantly reducing the need for infusion
May 23, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, BioMarin Pharmaceuticals announced at the World Federation of Hemophilia (WFH) World Congress in 2018 that the company's new treatment for hemophilia A, valoctogogene roxaparvovec (formerly known as BMN270), is open beta 1/2 clinical trial. The latest results in . The results of the trial showed that the therapy could eliminate the need for prophylactic clotting factor therapy in the second year after treatment, and that the patient did not have a spontaneous blood event, and the quality of life index continued to rise in the second year after receiving treatment.

Hemophilia A is a blood disorder in which factor VIII is absent due to genetic causes. It is the most common type of hemophilia, affecting men, and one in about 4000-5000 newborns. Due to the absence of coagulation factors, the patient's blood can not coagulate normally, and many patients often have spontaneous bleeding events. Currently, the standard treatment for patients with severe hemophilia A is intravenous injection of prophylactic factor VIII three times a week. But even so, there are still many patients with spontaneous bleeding.
Valoctogogene roxaparvovec, developed by BioMarin, is a gene therapy for the expression of factor VIII using the AAV vector. It has the advantage that patients may need only one treatment to obtain a gene that expresses factor VIII, eliminating the need for prolonged prophylactic clotting factor therapy. The therapy has been approved by the US FDA for breakthrough therapy.
In a clinical phase 1/2 trial called GENEr8-1, 40 patients with hemophilia A received treatment with valoctogogene roxaparvovec at a dose of 6x10^13 vector genomes/kg body weight (vector genome/kg, vg/kg). . The data released this time is the data of these patients for up to 104 weeks since receiving treatment. The results of the trial showed that this group of patients required significant and sustained reduction in bleeding events treated with factor VIII. The patient's Annualized Bleed Rate (ABR) was reduced by 97%, and there was no spontaneous bleeding event in the second year after treatment, and there was no form of bleeding in the targeted joint. 71% of patients did not have any bleeding events requiring treatment with factor VIII in the first year after treatment. This value increased to 86% in the second year after treatment, and the baseline value before treatment was 14 %. As of the 104th week of treatment, the patient's dose of factor VIII was reduced by an average of 96%. The Haemo-QoL-A index, which combines the six aspects of measuring the quality of life of patients with hemophilia, is comprehensively improved. Compared with the baseline value, the quality of life index increased by 17.3 points in the second year after receiving treatment, which is much higher than the clinical standard. The important difference is 5.2 points.
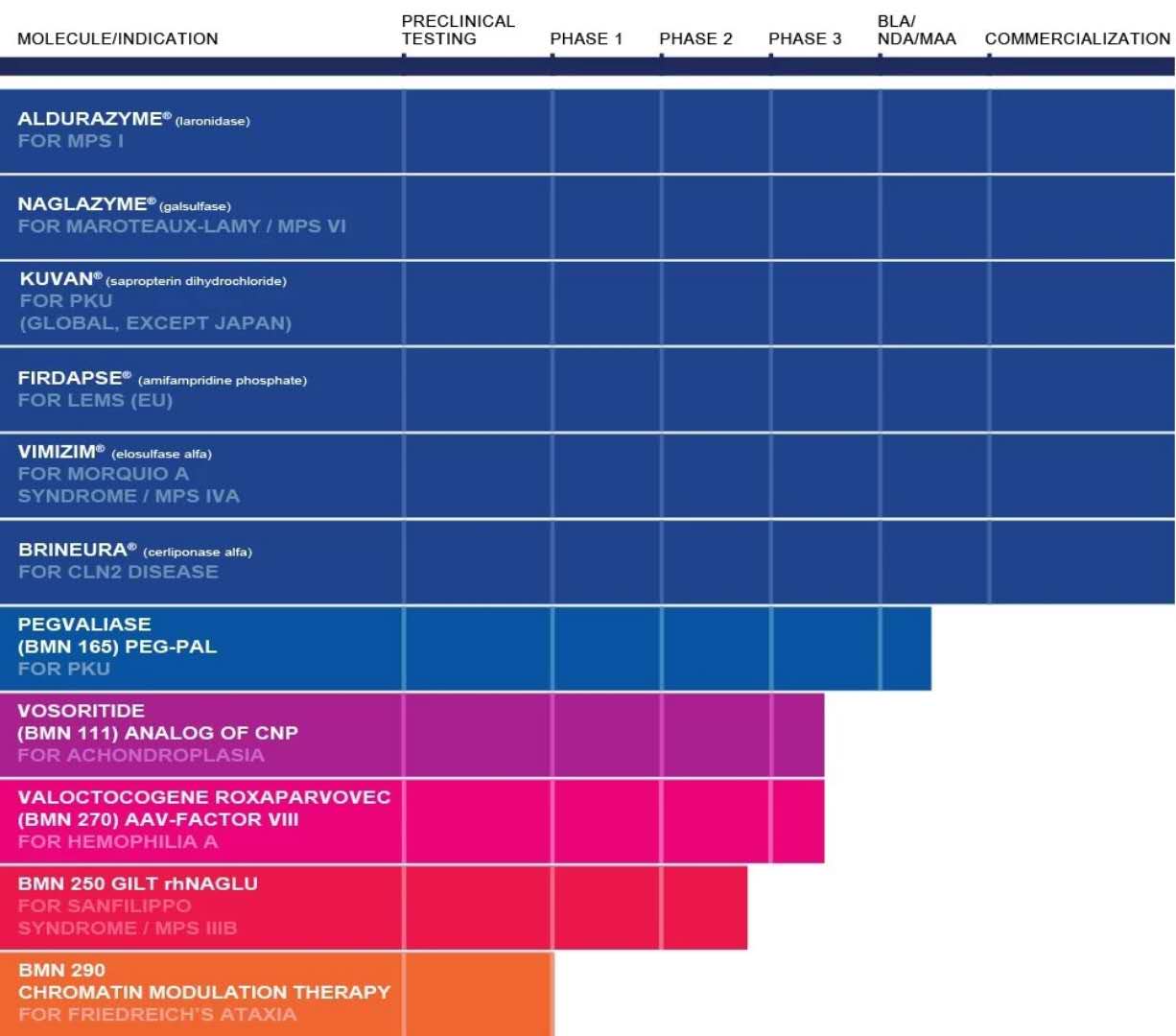
â–²BioMarin Pharmaceuticals research and development product line (Source: BioMarin Pharmaceuticals official website)
In this group of patients, the mean factor VIII levels in the blood of patients from 20 weeks to 104 weeks were always at normal or near normal levels, and no patients exceeded the upper limit of normal levels. In addition, valoctogogene roxaparvovec showed good tolerance, no patients developed inhibitors of foreign factor VIII, and no patients withdrew from clinical trials.
Based on positive data from the study, BioMarin decided to increase the number of patients involved in this clinical trial from 40 to 130. The trial protocol and objectives will be revised to test whether the valoctogogene roxaparvovec gene therapy at a dose of 6x10^13vg/kg is superior to the existing standard care therapy (prophylactic factor VIII therapy). The patient registration process for the revised GENEr8-1 clinical trial is expected to be completed in the first quarter of 2019.
"In order to obtain more data support and to deliver this treatment option to patients with severe hemophilia A as soon as possible, we plan to increase the number of samples from the GENEr8-1 study to demonstrate that the efficacy of this gene therapy is much higher than preventive. Coagulation factor therapy," said Dr. Hank Fuchs, president of global development at BioMarin.
We look forward to this new therapy to bring the gospel to patients as soon as possible!
Reference materials:
[1] BioMarin Provides 2 Years of Clinical Data in 6e13 vg/kg Dose from Ongoing Phase 1/2 Study in ValoctocogeneRoxaparvovec Gene Therapy for Severe Hemophilia A at World Federation of Hemophilia 2018 World Congress
[2] BioMarin Pharmaceuticals official website
The most common waxy and sweet corn market, waxy corn has higher nutrient content than ordinary corn, containing 70-75% starch (and almost all amylose), more than 10% protein, 4-5% fat and 2 % Of multivitamins have more grains, VA, VB1, and VB2 than rice with the highest content of protein, fat and VB2. Yellow corn also contains carotenoids, such as rice and wheat. The molecular weight of waxy corn starch is more than 10 times smaller than that of ordinary corn. The starch makes glutinous rice sticky and softer than ordinary hard corn. Its digestibility is more than 20% higher than that of ordinary corn, and it is suitable for people with irregular teeth. At the same time, the content of amylose (a kind of polysaccharide) is very high, which is not suitable for diabetics.
Waxy corn is also called sticky corn. The grains have rough, waxy endosperm, similar to shiny, transparent grains, such as hard and dented corn. Its chemical and physical properties are controlled by a recessive gene located on chromosome 9. 100% of the starch in the endosperm is amylose.


Waxy Corn Kernels,Yellow Corn Kernels,Non Gmo Corn Kernels,Yellow Waxy Corn Kernels
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com